
Data Management
Linical provides sponsors flexible data management solutions based on cost, timelines, study size and clinical strategy.
Clinical Data Management Services
High-quality data is central to your clinical trial’s success and regulatory approval. Data management (DM) must ensure the security of sensitive information related to patients and the clinical trial as well as compliance with established data privacy regulations. Linical’s DM capabilities are designed to give you accurate and actionable data, in a timely manner ensuring the integrity, reliability, and efficiency of information management.
We have the necessary experience, resources, and processes to guarantee efficient data management throughout the clinical trial. Our 20+ years of experience in clinical data management spans trials across North America, Europe, and Asia, and we have worked on numerous global projects ranging from phase I, II, and III clinical trials to late-phase, observational studies . Linical’s data management team has expertise using the industry’s top electronic data capture (EDC) platforms including Medrio, Viedoc, and Medidata Rave, allowing us the flexibility to meet the specific data management needs of our clients.
Services
Comprehensive End-to-End Data Management Capabilities
Explore
- CRF and database design
- Data management plan development and documentation
- Data validation and query management
- CCGs
- Medical coding (WHO Drug and MedDRA)
- SAE reconciliation
- External data transfers and reconciliation
- Ongoing data review, logging, and tracking
- Data validation and clean up
- Data quality assurance
- Data management reporting
- Secure central lab electronic data transfer and upload
- CDIS (Clinical Data Interchange Standards Consortium) datasets
- EDC Help Desk
- Database lock and audit
- Database release using sponsor format (SAS, CDISC (SDTM, define.xml), etc
- Medrio
- Viedoc
- Medidata Rave
Your Dedicated Partner for Data Management
Linical combines experience with technology and therapeutic knowledge to ensure efficient processes, actionable outcomes, and the highest quality data. No matter how big or small your project is, our DM team listens to your specific needs and offers recommendations that best fit your project, adding value every step of the way.
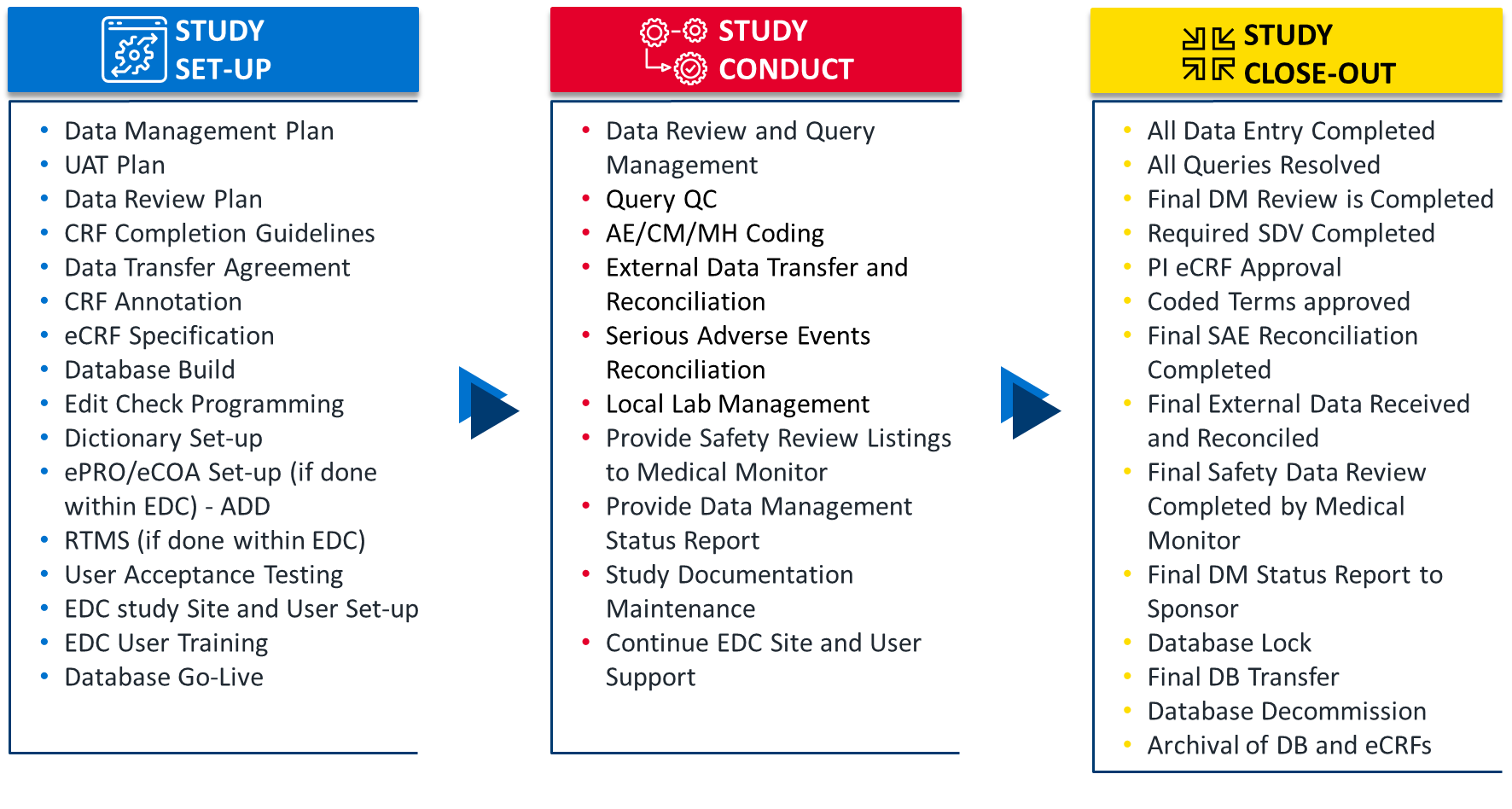
Data Management Oversight
Our Clinical Pharmacovigilance Services
This is optional text Lorem ipsum dolor sit amet, consectetur adipiscing elit, sed do eiusmod tempor incididunt ut labore et dolore magna aliqua. Ut enim ad minim veniam, quis nostrud exercitation ullamco laboris nisi ut aliquip ex
Lorem Ipsum has been the industry's standard dummy text ever since the 1500s, when an unknown printer took a galley of type and scrambled it to make a type specimen book.
Lorem Ipsum has been the industry's standard dummy text ever since the 1500s, when an unknown printer took a galley of type and scrambled it to make a type specimen book.
Lorem Ipsum has been the industry's standard dummy text ever since the 1500s, when an unknown printer took a galley of type and scrambled it to make a type specimen book.
Lorem Ipsum has been the industry's standard dummy text ever since the 1500s, when an unknown printer took a galley of type and scrambled it to make a type specimen book.
Lorem Ipsum has been the industry's standard dummy text ever since the 1500s, when an unknown printer took a galley of type and scrambled it to make a type specimen book.
Lorem Ipsum has been the industry's standard dummy text ever since the 1500s, when an unknown printer took a galley of type and scrambled it to make a type specimen book.
Why Linical?
The clinical development journey can be daunting and often leads to failure. With so much riding on your compound, you deserve the best chance at achieving your goals and positively impacting patients across the globe.
As a global, award-winning CRO, we can provide the strategy and support you need to position your clinical trial for success. We have an impressive track record of exceeding our enrollment goals and maintaining nearly a 90% client retention rate.
With our collaborative approach and commitment to quality, Linical expertly guides you through each step of the process, from early-phase research to large-scale global studies. With Linical, you can overcome obstacles, expedite timelines, save valuable money, and achieve your goals without compromising quality.
Successful clinical trials start with Linical.
Don’t let the complex clinical development journey hold you back. With Linical, you can overcome obstacles, save valuable time and money, and reach your goals.
01 Request a proposal
We start by listening to your needs and understanding your goals to ensure we’re the right CRO for you.
02 Get a plan for success
We propose solutions that proactively tackle obstacles, optimize your trial design, and position you for success every step of the way.
03 Execute with confidence
We’ll guide you through each phase of the process, offering personalized support and a full range of services to help you achieve a successful trial. We are not a “one size fits all” CRO.
.webp?width=518&height=780&name=cta-img%20(2).webp)